Which of the Following Is a Characteristic of Dispersion Forces
The F 2 molecule has a greater mass than the HF molecule has. 5Distinguishing characteristic of London dispersion forces a.
2 6 Intermolecular Force And Physical Properties Of Organic Compounds Organic Chemistry I
Hydrogen bonding only occurs when hydro- gen is covalently.

. The characteristics of two unknown intermolecular forces X and Y are compared in the table. Hence we can say that dispersion forces purely depend on the molecular. Dispersion forces and dipole-dipole forces.
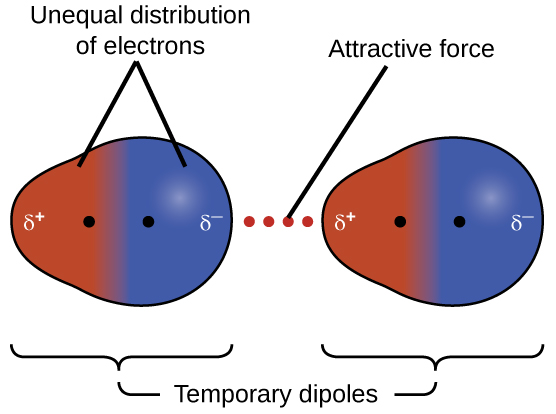
The electron cloud of the atoms are evenly distributed around the nucleus. Consider the intermolecular forces present in a pure sample of each of the following compounds. Liquid F 2 has weak dispersion force attractions between its molecules whereas liquid HF has strong ionic interactions between H and F - ions.
There is instantaneous dipole that influences neighboring substances to gain dipoles b. Are found in only nonpolar substances. The type of inter molecular forces exist in and the component uh and they have characteristics non polarity.
Dispersion forces occur in between the two atoms which have low molecular weight or two substances which are non-polar in type ie. It is weaker than a covalent bond. Foraging societies are nomadic groups.
Stronger dispersion forces result in lower boiling points. It is responsible for the unusual physical properties of water. So we can say that covalent bond ionic bond coordination bond are the intra-molecular force of attraction which form within a molecule.
Mixtures of ionic compounds and polar compunds. Which of the following is the characteristic of London dispersion forces. Stronger in linear molecules than spherical molecules of similar molar mass.
And therefore to a first approx to the atomic numbers of the constituent atoms in the molecule. Tell whether each of the following is a characteristic of London dispersion forces LD dipole-dipole interactions di-di or hydrogen bonding HB. In a larger atom or molecule the valence electrons are on average farther from the nuclei than in a smaller atom or molecule.
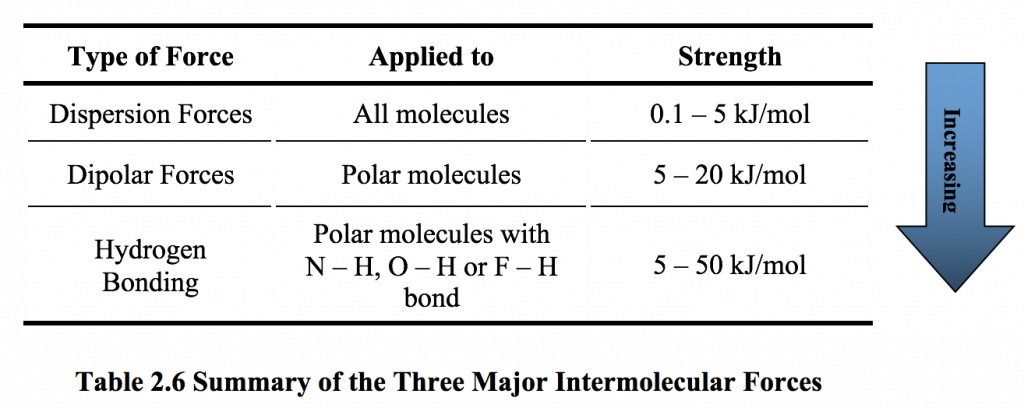
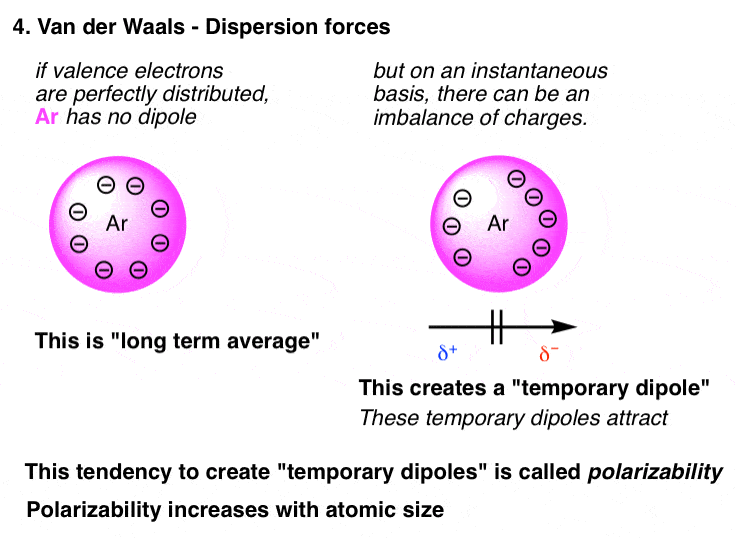
Temporary dipoles can occur in non-polar molecules when the electrons that constantly orbit the nucleus occupy a similar location by chance. Key Points London dispersion forces are weak intermolecular forces and are considered van der Waals forces. The strength of Van Der Waal Dispersion forces increases with the size shape molar mass and number of electrons in an atom or a molecule.
Foraging societies consist of small groups with ties based on. Molecular Size Dispersion forces are present between all molecules whether they are polar or nonpolar. The London dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles.
Which of the following is a characteristic of dispersion forces. Are a type of permanent dipole. There is permanent - and ends that participate in electrostatic attractions c.
London Dispersion Forces Definition. The electron cloud of the atoms are evenly distributed around the nucleus. Discuss the characteristics of dispersion forces.
Molecular solids have relatively low melting points. Molecules containing H bonded to F O or N. Dispersion forces are present in all molecules but non-polar molecules have only these forces.
This force is sometimes called an induced dipole-induced dipole attraction. Which of the following conclusions about the two intermolecular forces. The characteristics of two unknown intermolecular attractions A and B are compared in the table.
Answered expert verified. See the answer See the answer done loading. The London dispersion force is the weakest intermolecular force.
Identify the intermolecular forces that these compounds have in common. The electron cloud of the atoms is evenly distributed around nucleus. The forces of attraction between molecules which hold them together are called the intermolecular force of attraction.
The bigger the electron cloud the more polarizable it becomes and thus many electron xenon and. I will have London dispersion forces. F 2 is soluble in water whereas HF is insoluble in water.
Stronger dispersion forces result in lower boiling points. Which of the following is typically not a characteristic of a foraging society. Which of the following exhibit only london dispersion forces.
2 Show answers Another question on Geography. There is permanent - and ends that participate in electrostatic attractions C. Rank the following types of intermolecular forces in general order of decreasing strength strongest to weakest.
Which of the following conclusions about the two attractions is correct. A represents ion-dipole interactions and B represents hydrogen bonding. And this is nicely indicated by the Noble Gas series for which London forces are the ONLY intermolecular here interatomic force.
Larger and heavier atoms and molecules exhibit stronger dispersion forces than smaller and lighter ones. It is stronger than other dipole-dipole interactions. Are a type of permanent dipole.
Occurs between molecules with instantaneous dipoles Occurs between molecules with permanent dipoles and ions. It is considered as the weakest of all intermolecular forces. Stronger in linear molecules than spherical molecules of similar molar mass.
Dispersion forces are a basic force between two molecules or atoms but it is the weakest attractive force in between them called dispersion forces. So this non polarity characteristics is produced due to the negligible negligible electronic activity difference. 1 point A represents London dispersion forces and B represents hydrogen bonding.
There is permanent - and ends that participate in electrostatic attractions c. Are found in only nonpolar substances. Ion-dipole hydrogen bonding dipole-dipole dispersion.
Molecular solids are usually excellent conductors of electric current. It can occur when hydrogen is covalently bound to very electronegative elements liks F Cl Br and I. The binding forces in molecular solids are dispersion forces or dispersion forces and dipole-dipole interactions.
The atoms of two neighbouring molecules participate in give and take of electrons. Geography 21062019 1940. Distinguishing characteristic of London dispersion forces a.
These forces are weaker than intermolecular forces. There is instantaneous dipole that influences neighboring substances to gain dipoles b. _ the weakest intermolecular force the strongest intermolecular force the attraction between the positive end of one permanent dipole and the negative end of another the attraction between temporarily induced dipoles the.

Cement Thickener Concrete Mortar Chemical Additives It Is A Semi Synthetic Inactive Viscoelastic Polymer With High Water Retention Dispersion Excellent Fine 2022

12 6 Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts

Selina Concise Chemistry Class 6 Icse Solutions Chapter 5 Pure Substances And Mixtures Separation Of Mixt Chemistry Lessons Teaching Chemistry Chemistry Class

Quickstudy Bio Lab Basics Laminated Study Guide Essay Outline Sample Essay Outline Study Guide

London Dispersion Forces Definition Causes Examples

Chapter 1 The Solid States Class 12 Chemistry Handwritten Notes Pdf In 2022 Chemistry Notes Handwritten Notes Chemistry

Difference Between Solid Liquid And Gas In Tabular Form States Of Matter Simplified Solid Liquid Gas States Of Matter Intermolecular Force

Pin By Kelvin Hung On Physics Maxwell Relations Relatable Chemistry Classroom

Intermolecular Forces Magic Trick Youtube Intermolecular Force Teaching Chemistry Chemistry Teacher

Dispersion Force An Overview Sciencedirect Topics
The Four Intermolecular Forces And How They Affect Boiling Points

Metallic Bond Metallic Bonding Bond Ionic Bonding

Semester Review Lab Day 1 Borax Ornaments Chemistry Classroom Borax Crystal Ornaments Physical Science High School

Thalidomide How Stereoisomers Can Have Complete Different Properties Chemistry Lessons Chemistry Lesson
10 1 Intermolecular Forces Chemistry

Pin By Obul Reddy On Aplus Topper Intermolecular Force Pressure Change

11 7 Structure Of Solids Chemistry Libretexts Unit Cell Crystalline Solid Intermolecular Force

The Arrangement Of Atoms In Crystalline Solids Crystalline Solid Coordination Number Cell Parts
Comments
Post a Comment